Matters of size: when is the cornea too large?
The cornea is the most significant refractive structure of the eye, contributing two-thirds of the eye’s refractive power, the remainder coming from the crystalline lens. Normal values for corneal diameters in adults have means ± SD of 11.71 ± 0.42mm horizontally and 10.63 ± 0.63mm vertically, while normal values for anterior corneal curvature and central corneal thickness range from 38.9 to 47.8 diopters (7.06 to 8.66mm) and 490 to 620µm respectively. These corneal parameters tend to vary with age, gender and ethnicity.
Accurate measurement of the corneal diameter is important in the diagnosis of corneal disorders of size, such as megalocornea or macrocornea. An abnormally large cornea is typically characterised by corneal diameters greater than 12.5mm. Large corneas in conditions such as in megalocornea, anterior megalophthalmos and buphthalmos may be associated with myopia, cataracts, astigmatism and glaucoma. Larger corneal diameters (0.5-0.6mm larger than controls in the same study), but within the normal range, have been reported in keratoconus and granular corneal dystrophies, while diameters have been noted to be 0.2mm smaller in Fuchs’ endothelial corneal dystrophy.
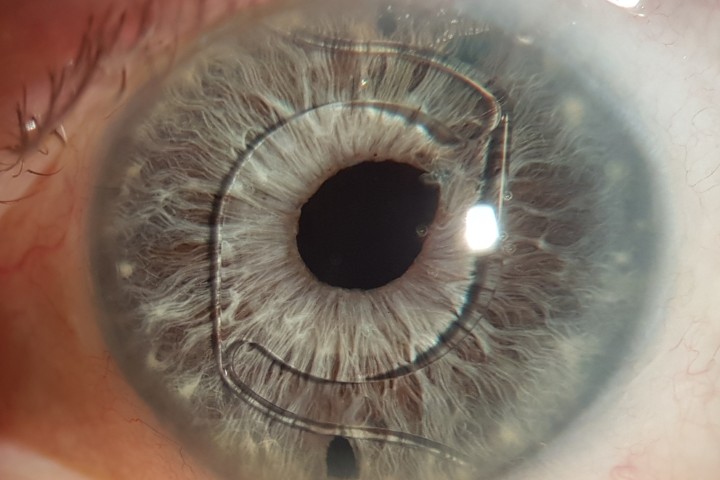
Historically, there has been a lack of clarity in how measurement of the human corneal diameter should be best obtained. The corneal diameter as determined by the horizontal white-to-white (WTW) has diverse clinical applications (Fig 1) and is relied upon for haptic size calculation in relation to a variety of intraocular lenses. WTW measurement can either be performed manually with calipers intraoperatively, with the inbuilt scale on some slit-lamps, or via automated anterior segment imaging devices such as: anterior segment optical coherence tomography (AS-OCT); ultrasound biomicroscopy (UBM); IOL Master (Zeiss); or computerised corneal tomography.
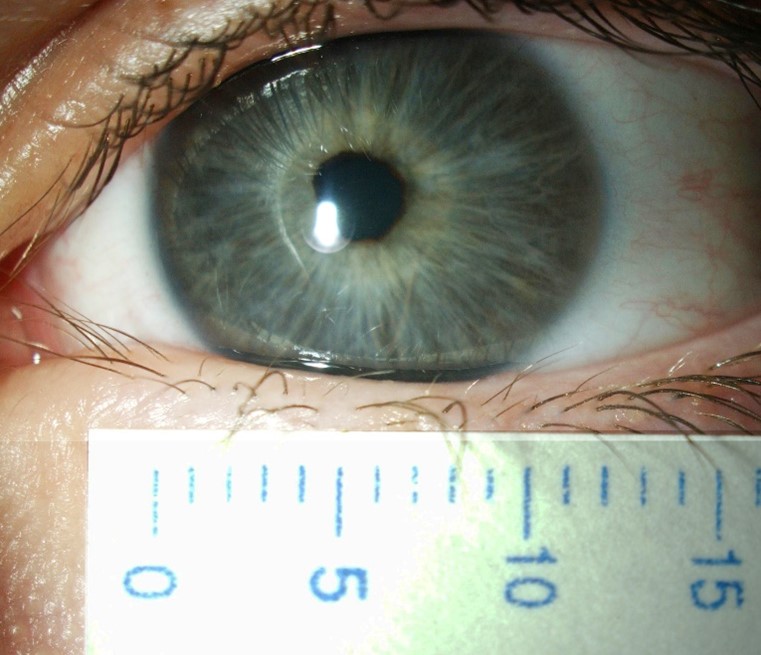
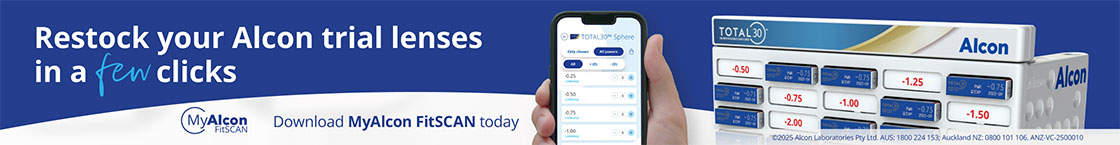
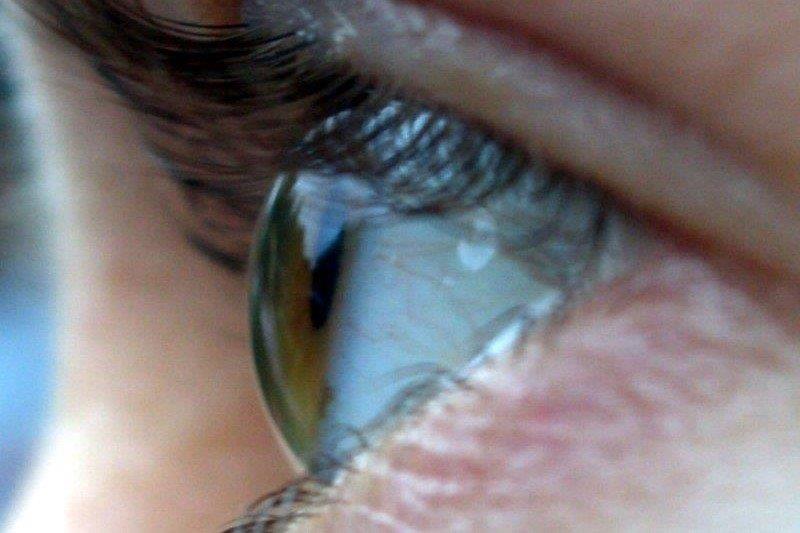
Fig 1. Megalocornea with an overall manual white-to-white
measurement slightly larger than 12.5mm
Unfortunately, exact estimation of the normal horizontal corneal diameter range is highly subjective and imprecise. Measurement of the horizontal visible iris diameter (HVID), which is defined as the horizontal diameter of the iris within the clear corneal zone, provides limited acknowledgement of the limbal width and its involvement as part of the corneal diameter. Limbus to limbus measurement serves as a more accurate indicator but defining the corneal side of the limbal zone and peripheral limit of the limbus along the anterior ocular surface can be challenging, especially given the gradual loss of corneal transparency across the limbus to the opaque sclera (Fig 2).
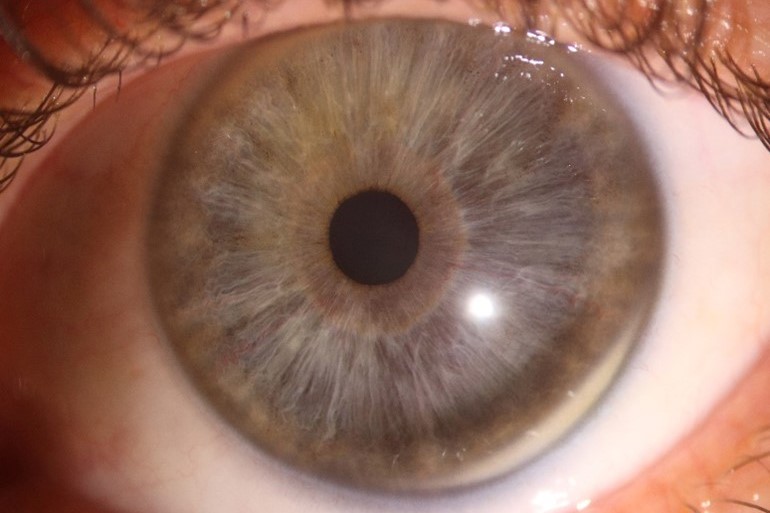
Though more objective measurements can be made with modern, automated instrumentation, the relative absence of structural definition and low magnification resolution raises doubts regarding the accuracy of many results. Typically, a topographic assessment of corneal diameter with a sulcus-to-sulcus measurement tends to derive a larger corneal value as it will involve at least part of the limbus. Furthermore, limbal widening or presence of arcus senilis with age might result in inaccurate topography measurement (Fig 3). Similarly, precision of OCT is limited by low resolution at low magnification, which inhibits accurate identification of anatomical reference points.
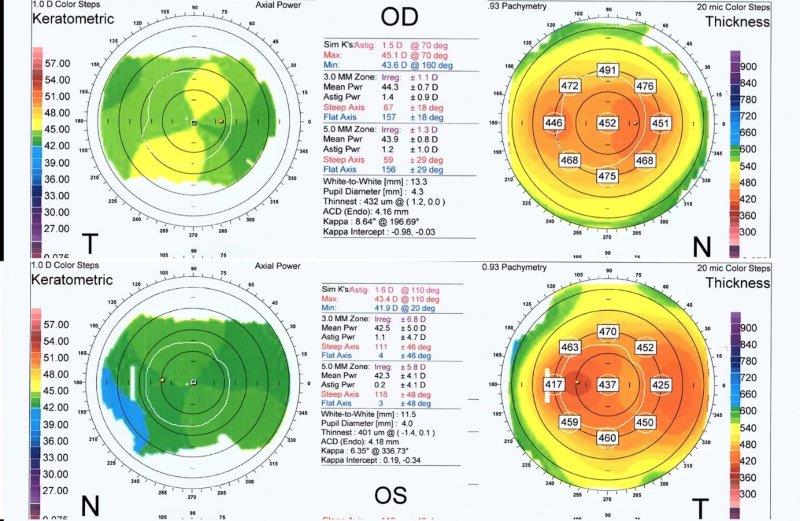
Fig 3. Corneal topography of the right (OD) and left (OS) eye (lower images) in anterior megalophthalmos. Keratometric power (upper and lower left) is within normal limits with OD max of 45.1 diopters and OS max of 43.4 diopters. Astigmatism is mild at 1.5 diopters OD and 1.6 diopters OS. Despite both corneas being the same size clinically, the
topographic white-to-white measurement is 13.3mm OD and only 11.5 mm OS, highlighting potential inaccuracy. Corneal thickness maps (upper and lower right) demonstrate a regular pattern of uniform thinning from centre to periphery in the region of 450μm in each eye (unlike the grossly asymmetric thinning associated with keratoconus)
In this article, we highlight a few illustrative clinical cases which may be encountered in clinical practice.
Megalocornea and anterior megalophthalmos
Megalocornea, also known somewhat interchangeably as anterior megalophthalmos, X-linked megalocornea and microcornea, is a bilateral, nonprogressive and rare hereditary disorder which is characterised by a corneal diameter larger than 12.5mm in the absence of elevated intraocular pressure. It is typically associated with an X-linked recessive inheritance (CHRDL1 gene on Xq23) which accounts for the male preponderance of the disorder.
Common clinical features include iris atrophy, iridodonesis and subluxation or dislocation of the crystalline lens. It can occur in isolation or associated with various ocular and systemic abnormalities, such as mosaic dystrophy, arcus senilis, Marfan syndrome and Neuhauser syndrome.
Typically, the term megalocornea refers to corneal changes in isolation, whereas anterior megalophthalmos refers to the disease when associated with an enlarged crystalline-lens-iris-diaphragm and ciliary ring in the absence of features suggestive of congenital glaucoma. It is commonly characterised by anterior embryotoxon, iris hypoplasia, myopia, cataract, glaucoma and lens subluxation or dislocation. X-linked recessive is usually the most common mode of inheritance. Down syndrome, Marfan syndrome and mucolipidosis type 2 have been found to be associated with this disorder (Fig 4).
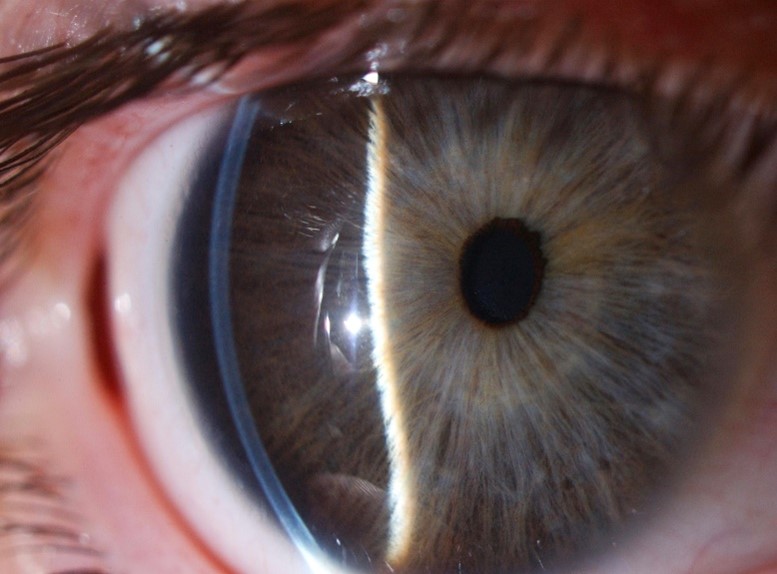
Fig 4. A case of anterior megalophthalmos with a uniform, modestly thin cornea of large diameter, with a normal corneal curvature, anomalously deep anterior chamber and a degree of iris retroflexion
Buphthalmos
Buphthalmos should be in the differential of any case of megalocornea. It refers to progressive globe enlargement associated with persistently elevated intraocular pressure between the end of foetal life and the third year after birth. It is a typical, but not pathognomonic, sign of primary congenital glaucoma. Classical manifestations include epiphora, photophobia, blepharospasm, corneal oedema, striae of Descemet’s membrane (Haab’s striae), anterior scleral thinning, anomalously deep anterior chamber and progressive glaucomatous optic atrophy. It may occur sporadically or be inherited in an autosomal recessive pattern (CYP1B1 and LTBP2 genes) (Fig 5).
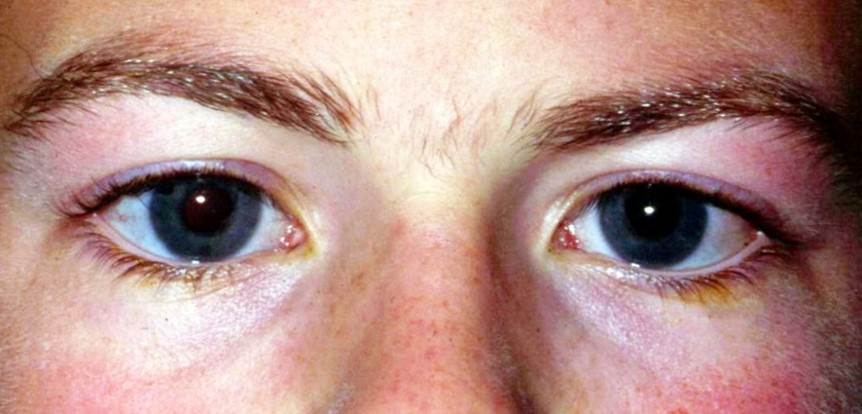
Fig 5. Buphthalmos in congenital glaucoma with a corneal diameter of 13.5mm,
representing more than 50% of the normal palpebral fissure length in adults (approx.
25mm)
Keratoglobus
Keratoglobus is a rare, bilateral, noninflammatory corneoscleral ectasia characterised by globular protrusion of the cornea without enlargement of the corneal diameter. There is generalised corneal thinning with marked reduction in stromal thickness, especially peripherally, which makes surgical management difficult. There may be considerable overlap of features with megalocornea and the two may be mistaken clinically, especially in cases without a family history.
Additional features of keratoglobus may include central epithelial hyperplasia, scarring and neovascularisation of the stroma, and disruptions in Descemet’s membrane. It can present either in the congenital form, which is associated with blue sclera syndrome, Ehlers-Danlos type VI and Leber congenital amaurosis, or the acquired form, associated with vernal keratoconjunctivitis and chronic marginal blepharitis. There are limited reports of the genetics of keratoglobus and no definite inheritance pattern has been described. Severe keratoconus can also take on a ‘globular’ form, but unlike keratoglobus this is typically a progressive disease, associated with a normal corneal diameter, asymmetric corneal thinning, severe irregular astigmatism, striae and corneal scarring (Fig 6). Table 1 below highlights the differentiating features between these four conditions.
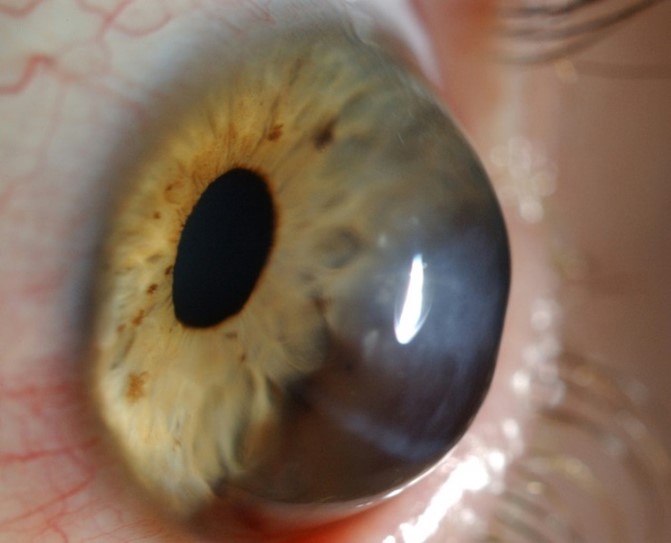
Fig 6. Keratoconus in globus form, with normal corneal diameter, marked protrusion, central scarring and variable extreme, corneal thinning from limbus to limbus
Conclusion
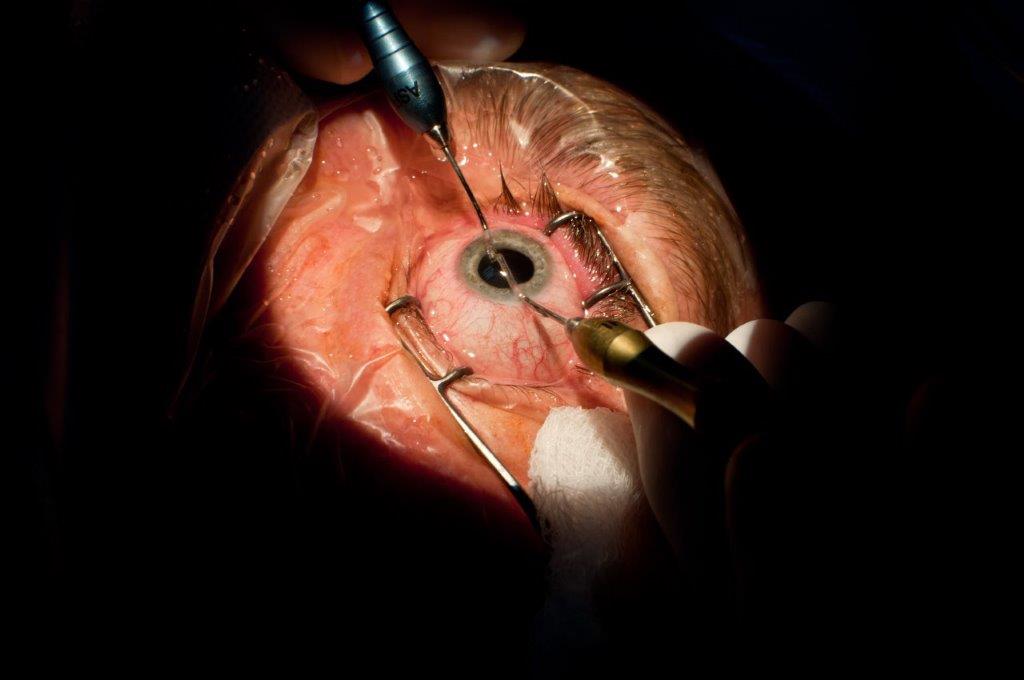
There is a lack of agreed and precise information on what the average corneal diameter should be, its normal range and the best method of measurement. Large corneal diameters are evident in cases of megalocornea and anterior megalophthalmos, which are rare disorders where patients are predisposed to cataract development, amongst other complications, and management can be challenging, especially during cataract surgery (Fig 7). Megalocornea is part of a spectrum that includes anterior megalophthalmos but is quite distinct from buphthalmos, and although it has overlapping features with keratoglobus it is unlike severe keratoconus. Measurement of cornea parameters is recommended in adults and children with larger eyes and corneal diameters of 12mm or more should raise suspicions and warrant further investigations. Despite the constellation of complications associated with these rare disorders, clinical outcomes are often favourable.
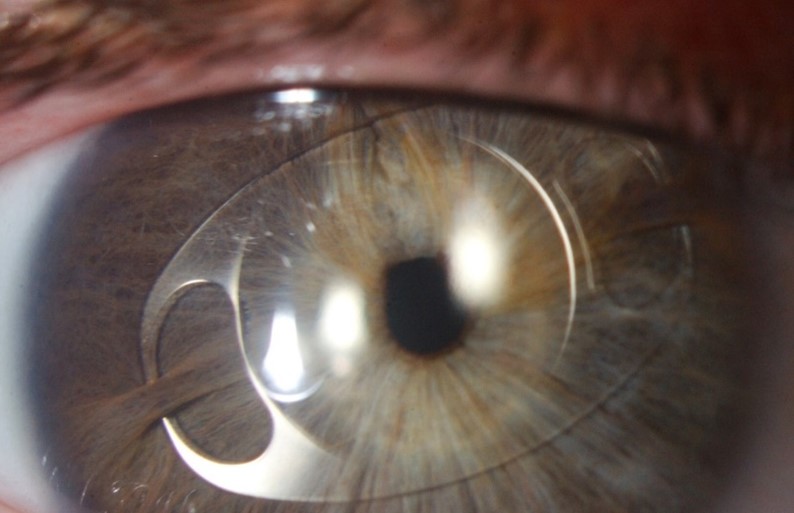
Fig 7. Management of cataract in anterior megalophthalmos is complicated by poor zonular support and fragility of lens structures often leading to alternative options such as use of iris clip intraocular lenses
Bibliography
- Budak K, Khater TT, Friedman NJ, Holladay JT, Koch DD. Evaluation of relationships among refractive and topographic parameters. Journal of Cataract & Refractive Surgery. 1999 Jun 1;25(6):814-20.
- Rüfer F, Schröder A, Erb C. White-to-white corneal diameter: normal values in healthy humans obtained with the Orbscan II topography system. Cornea. 2005 Apr 1;24(3):259-61.
- Khng C, Osher RH. Evaluation of the relationship between corneal diameter and lens diameter. Journal of Cataract & Refractive Surgery. 2008 Mar 1;34(3):475-9.
- Kiely PM, Smith G, Carney LG. Meridional variations of corneal shape. American Journal of Optometry and Physiological Optics. 1984;61(10):619-26.
- Bergmanson JP, Martinez JG. Size does matter: what is the corneo‐limbal diameter?. Clinical and Experimental Optometry. 2017 Sep;100(5):522-8.
- Pinero DP, Puche AB, Alió JL. Corneal diameter measurements by corneal topography and angle-to-angle measurements by optical coherence tomography: evaluation of equivalence. Journal of Cataract & Refractive Surgery. 2008 Jan 1;34(1):126-31.
- Hall LA, Hunt C, Young G, Wolffsohn J. Factors affecting corneoscleral topography. Investigative Ophthalmology & Visual Science. 2013 May 1;54(5):3691-701.
- Messina M, Ross AR, Pocobelli G, Said DG, Dua HS. Cataract surgery with intraocular lens implantation in 3 brothers with megalocornea: Long-term follow-up. Journal of Cataract & Refractive Surgery. 2018 Mar 1;44(3):399-402.
- Webb TR, Matarin M, Gardner JC, Kelberman D, Hassan H, Ang W, Michaelides M, Ruddle JB, Pennell CE, Yazar S, Khor CC. X-linked megalocornea caused by mutations in CHRDL1 identifies an essential role for ventroptin in anterior segment development. The American Journal of Human Genetics. 2012 Feb 10;90(2):247-59.
- Kumawat D, Alam T, Sahay P, Chawla R. Ocular abnormalities and complications in anterior megalophthalmos: a case series. Eye. 2019 May;33(5):826-32.
- Lewis CJ, Hedberg-Buenz A, DeLuca AP, Stone EM, Alward WL, Fingert JH. Primary congenital and developmental glaucomas. Human Molecular Genetics. 2017 Aug 1;26(R1):R28-36.
- Meghpara B, Nakamura H, Vemuganti GK, Murthy SI, Sugar J, Yue BY, Edward DP. Histopathologic and immunohistochemical studies of keratoglobus. Archives of ophthalmology. 2009 Aug 1;127(8):1029-35.
- Ku JY, Grupcheva CN, Fisk MJ, McGhee CN. Keratoglobus and posterior subcapsular cataract: surgical considerations and in vivo microstructural analysis. Journal of Cataract & Refractive Surgery. 2004 Jan 1;30(1):237-42.
Dr Jie Zhang is a senior HRC research fellow and scientist with interests in both laboratory and clinical research.
Dr Aaron Ong is a clinical research fellow pursuing ophthalmology.
Both are based in the Department of Ophthalmology, headed by Professor Charles McGhee, at the University of Auckland.