OSL: Guiding eye drop choice
Clinicians and patients are faced with an overwhelming choice of artificial tear supplements as a therapy for their dry eye disease (DED). Focus was once on the lacrimal gland and aqueous tear deficiency, however meibomian gland dysfunction and tear film lipid deficiency are increasingly recognised as playing an important role in DED pathophysiology.
In the wake of this better understanding, a number of eyedrop formulations now incorporate lipids to address evaporative forms of the disease, while aqueous-based drops continue to be used to compensate for lacrimal gland insufficiency¹. However, sound scientific evidence to guide eye care professionals in their targeted choice of artificial tear supplements according to the spectrum of disease subtypes is limited, with most studies investigating only short-term effects of tear film supplementation².
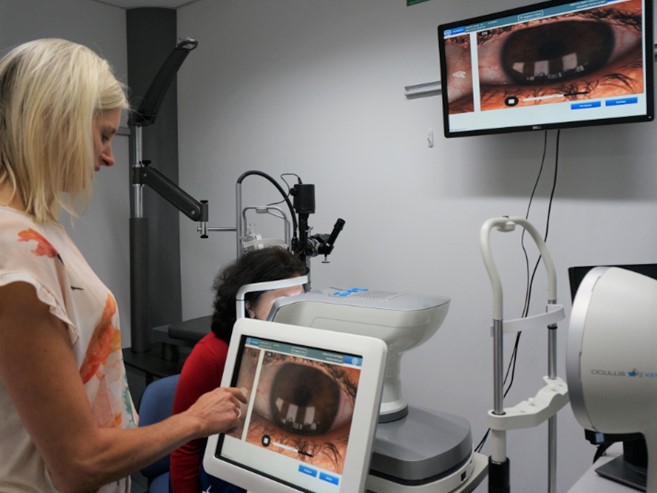
To address this knowledge gap, a collaborative research project led by the Ocular Surface Laboratory (OSL) and involving research centres in the UK, Canada and Australia was established with investigator-initiated trial funding awarded by Alcon Laboratories (Australia). A six-month, multicentre, double-masked, randomised controlled trial was conducted to evaluate the efficacy of lipid and non-lipid based artificial tear supplements in the management of dry eye disease. In total, 99 participants were randomised to receive four times daily topical bilateral application of either the lipid-based or non-lipid-based drop, over a period of six months. Symptoms and clinical signs of tear film and ocular surface changes were assessed each month against validated, global consensus diagnostic markers³.
Improvements in signs and symptoms demonstrated distinct time courses, with symptomatic relief apparent as early as the one-month visit, while more profound, structural improvements in tear film and ocular surface integrity were observed after several months of continued use. Both lipid and non-lipid preparations were efficacious. Patients exhibiting tear film lipid deficiency, however, preferentially benefited from the lipid-based preparation.
While artificial tear supplementation for DED is recognised to offer a largely palliative effect, the results of this study suggest there is benefit to be gained from sustained, long-term use of eye drops, presenting a compelling argument for regular application of drops as a preventative strategy against the progression of DED.
This novel study represents a valuable step towards mapping therapeutic strategies according to disease subtype and severity, while highlighting the importance of long-term, clinically relevant follow-up and robust study design in helping to address critical gaps in our understanding of DED. Look out for more results from this study in a future issue of NZ Optics.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017; 15: 276–283.
2. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database of Systematic Reviews;2016, Issue 2. Art. No.: CD009729.
3. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf 2017; 15: 539–574.
Dr Ally Xue is a post-doctoral research fellow at Auckland University’s Department of Ophthalmology. Also acknowledged in this study is fellow post-doctoral researcher Dr Alex Müntz.