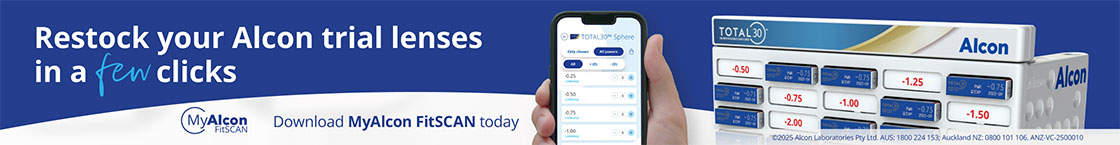
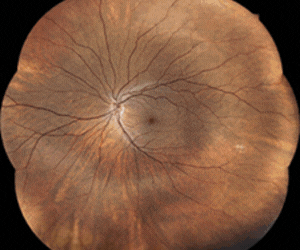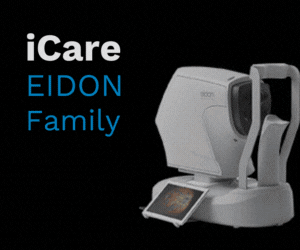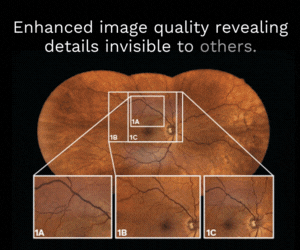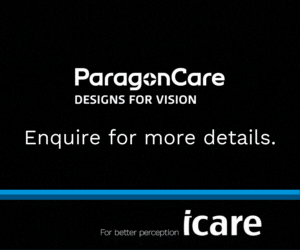
Progress for retinal prosthesis system
Popular press trumpeted the first implant of a “bionic eye” for patients with dry age-related macular degeneration when doctors performed the first implant and activation of the Argus II Retinal Prosthesis system earlier this year.
The bionic eye part is debatable, but the operation--performed at the Manchester Royal Eye Hospital in June by Dr Paulo Stanga--was a step forward for the Argus II system, which was originally tested, and approved for restoring some vision in patients with retinitis pigmentosa (RP) in the US in 2013 and Europe in 2011.
The Argus II System has now been implanted in 150 patients in the US, Canada, France, Italy, Germany, the Netherlands, Saudi Arabia, Spain, Switzerland and the UK. The Manchester implant was part of a feasibility trial for evaluating Argus II for treating late-stage Dry AMD.
While Second Sight Inc.--the California based company behind the Argus II system--has not been the only company in the last 15 years to pursue retinal implant systems, it appears to have gone the furthest, at least as far as feasibility and clinical trials, and regulatory approval.
Argus II is comprised of a small electronic device implanted in and around the eye, a small video camera attached to a pair of glasses, and a video processing unit worn or carried by the patient. The video processing unit receives images captured by the camera, and turns them into signals transmitted to the implant wirelessly. The implant transmits the signals to the retina in electrical pulses, which are intended to bypass damaged photoreceptors by stimulating the retina’s remaining cells. The system creates the perception of patterns of light which patients can learn to interpret as visual patterns.
According to an announcement released by Second Sight, the Argus II Manchester implant was the first of a larger study to expand the application of the system. Eligibility for this study includes patients 25 to 85 years of age with advanced dry AMD, some residual light perception and a previous history of useful form vision. Study subjects will be followed for three years to evaluate safety and utility of the system on visual function.
The first recipient of the Argus II in the study was Ray Flynn, 80, of Manchester who had lost his central vision to AMD, but had some peripheral vision. According to reports, Flynn was able to detect the pattern of horizontal, vertical and diagonal lines on a computer screen using the implant in a test just two weeks after surgery.
In its approval of the device for use in up to 4,000 procedures per year, the FDA in 2013 stated it had reviewed data that included a clinical study of 30 participants with RP who received the Argus II Retinal Prosthesis System. Investigators monitored participants for adverse events related to the device or to the implant surgery and regularly assessed their vision for at least two years after receiving the implant.
Results from the clinical study show that most participants were able to perform basic activities better with the Argus II than without it including locating and touching a square on a white field, detecting motion direction and recognising large letters, words, or sentences, among other tests.
Following the implant surgery, 19 of the 30 study patients experienced no adverse events related to the device or the surgery. However, 11 study subjects experienced a total of 23 serious adverse events, including erosion of the conjunctiva, dehiscence, retinal detachment, inflammation, and low intraocular pressure.