Cataract laser reversal met with scepticism
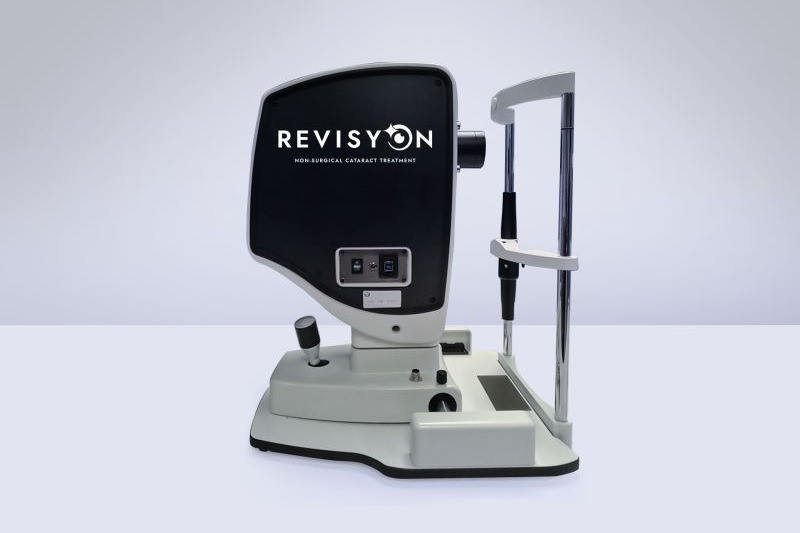
Edinburgh Biosciences, the UK developer of Revisyon (formerly Ledinbio), has claimed it is the world’s first non-invasive, one-stop diagnosis and treatment solution to reverse cataracts. The company recently announced it had secured a £2.3m (NZ$5.2m) loan investment from contact-lens manufacturer Contamac to support its commercial launch, with regulatory approval pending.
Founded by the late physicist Professor Desmond Smith, Edinburgh Biosciences says on its website that Revisyon uses specific wavelengths of light to trigger chemical changes in lens proteins, restoring their natural shape. “This transformation eliminates light scatter and reflection, letting light pass through effortlessly for brighter, sharper vision,” it states.
The company has been reported in the UK as saying it is anticipating obtaining a UK Conformity Assessed mark by the end of 2025, and that Revisyon could “shift cataract treatment away from the operating theatre and onto the high street, shortening the care pathway for millions awaiting surgery”.
According to Edinburgh Evening News, Edinburgh Biosciences (erstwhile) CEO Graham Bell presented initial clinical trial findings at the medtech conference LSX World Congress in London on 29 April this year, including results showing Revisyon improves visual acuity.
New Zealand context
Edinburgh Biosciences positions Revisyon for patients diagnosed with cataracts but not yet ready for, or wishing to avoid, surgery. It suggests potential appeal for those on long waiting lists or preferring fast, recovery-free treatment delivered in community optometry settings.
With 20 New Zealand regions still maintaining higher thresholds for cataract surgery, according to Official Information Act data reported by 1News, delays in surgery persist and questions remain about whether technologies such as Revisyon could offer an alternative.
Christchurch ophthalmologist Dr Zea Munro said she is following Revisyon developments closely. “I have been trying to follow any results. Unfortunately, as far as I am aware, they have not yet published their exact method or results yet.”
At the time of printing, the Edinburgh Biosciences website said of Revisyon, “We are currently carrying out clinical trials, which are showing that visual acuity has been improved in moderate cataracts with patients reporting significant improvements in their quality of life.” No peer-reviewed data have yet been published.
Specialist scepticism
Meanwhile, other New Zealand specialists remain cautious. Hamilton surgeon Associate Professor James McKelvie said he is “highly dubious” it would work. “At best it may be a temporary solution that could be very expensive.” He also questioned company comparisons with cataract surgery, noting some were “incorrect or overstated”.
Eye Institute’s Dr Peter Hadden also expressed doubts. “Thanks to my own work with penguins (whose corneal opacity is comparable to human corneas), I know that light less than 320nm cannot pass through the cornea; this is the high-energy light that could potentially change molecules (in the way Edinburgh Biosciences describes).” If used, such light could risk DNA mutations and cancer, he warned. “Visible and near-visible light – what we see and which does penetrate the cornea – if anything, causes cataracts, while longer wavelengths of light do not carry enough energy. Above 2,500nm the cornea is opaque to it anyway.” Like others, he stressed the need for double-blind, placebo-controlled trials in peer-reviewed journals before the technology could be endorsed.
Concerns about the technology extend beyond New Zealand. A LinkedIn post by Bell promoting Revisyon drew criticism from UK medical-device executive Alex Tangen, who argued the device “is in all likelihood at least a class 2M laser product” and warned that positioning it for use by optical dispensers could trigger strong resistance from surgeons and regulatory bodies. “The liability insurance alone could be very tricky to navigate.”
Edinburgh Biosciences did not publicly respond to the post not to requests for comment for this article.