DMO drop’s trial success
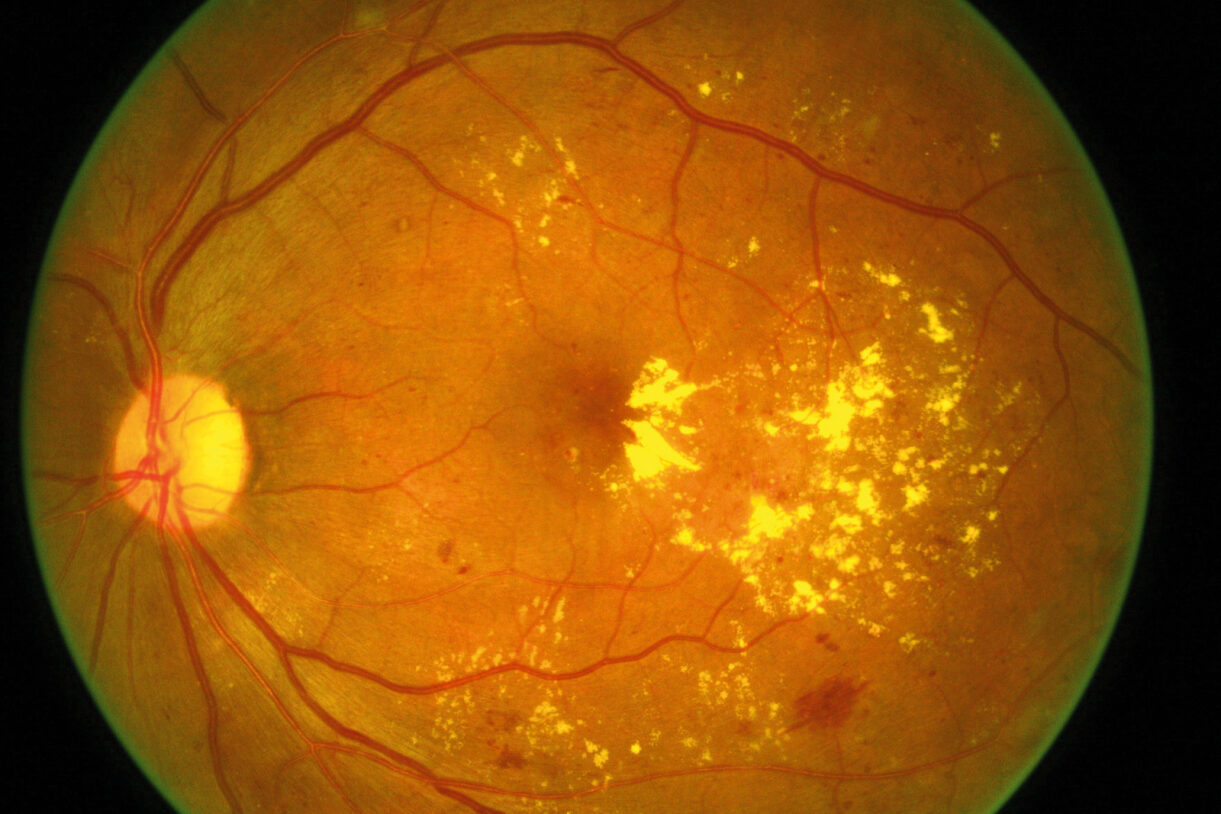
Exonate reported EXN407, its topical treatment for diabetic macular oedema (DMO) and diabetic retinopathy, met all primary endpoints in its phase 1b/2a trial.
The trial’s DMO patients were treated twice a day for three months with either EXN407 or placebo. EXN407-treated patients showed highlighted signals of biological activity (macular thickness reduction and decrease in retinal vascular leakage) and only mild adverse events, none of which led to treatment discontinuation, the company said.
The trial data validate the hypothesis that modulating VEGF splicing can lead to clinical benefits, said Exonate CEO Dr Catherine Beech. “We are excited to progress to the phase 2 trial in patients with severe DMO this year and welcome enquiries by potential partners for the programme.”