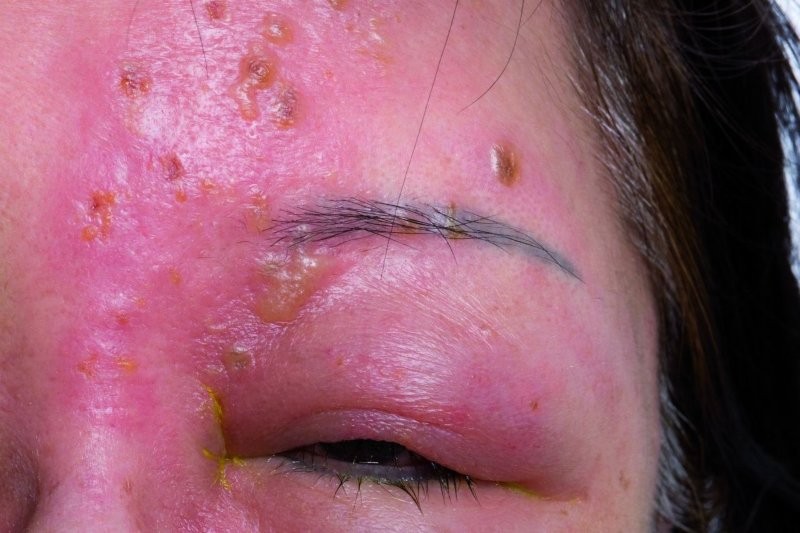
Valacyclovir reduces eye shingles pain
An international clinical trial, that included Auckland University researcher Dr Rachael Niederer, has found that one year of suppressive treatment with valacyclovir was associated with a lower dosage of neuropathic pain medication from herpes zoster ophthalmicus (HZO).
The Zoster Eye Disease Study (ZEDS), published in JAMA Ophthalmology, involved 527 adults across 95 sites in the US, Canada and New Zealand. Participants received either 1000 mg daily of valacyclovir or a placebo for 12 months. Overall, valacyclovir did not significantly reduce the prevalence of postherpetic neuralgia (PHN) at 12 months, the authors reported. However, they noted secondary analyses showed those under 60 years at HZO onset and who had chronic disease (six months or longer) reported less pain, shorter duration of symptoms, and used lower doses of neuropathic pain medications compared to the placebo group.
Authors say the results may guide clinicians in tailoring treatment for younger HZO patients with persistent symptoms and that the study highlights the potential of long-term antiviral therapy to improve quality of life for people affected by this condition.
They added that the recommended way to prevent postherpetic neuralgia is to prevent herpes zoster by vaccination with the recombinant zoster vaccine. “Underuse of vaccination remains a public health problem, given the increasing burden of herpes zoster for individuals younger than 60 years of age and the growing use of immunosuppressive medication for immune-mediated diseases. Because the use of valacyclovir in the current study was not superior in reducing the prevalence of postherpetic neuralgia, vaccination continues to be the most effective intervention,” the researchers said.