Fuchs’ endothelial corneal dystrophy: beaten metal to rock
Fuchs’ endothelial corneal dystrophy (FECD) was first described in 1910 by Ernst Fuchs and is the most common posterior corneal dystrophy. FECD is a progressive dysfunction of the corneal endothelium that may eventually result in corneal oedema and reduced vision.
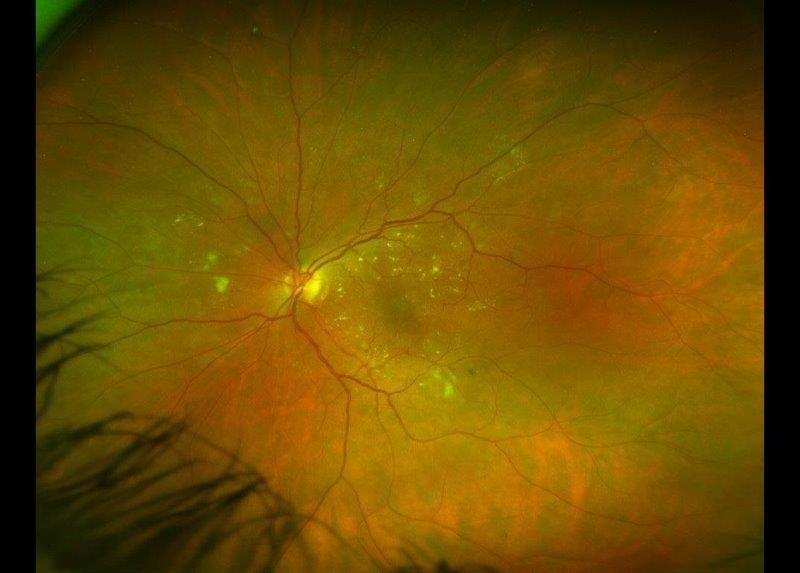
Since the first description of FECD in 1910, the aetiology and clinical signs have been elaborated upon in great in detail and management options have progressed immensely. The initial clinical finding, corneal guttae (the excrescences or drop-like lesions formed by stressed endothelial cells on Descemet’s membrane that create the classic beaten-metal appearance), are visible on slit-lamp examination, specular and in vivo confocal microscopy. Guttae usually manifest in the fourth decade of life and progress as the disease advances (Fig 1). Typically, it can take 20-30 years before intervention is required. However, FECD affects all corneal layers and worsening stromal oedema leads to the development of subepithelial and epithelial corneal bullae, which can rupture, causing pain and photophobia. Corneal transplantation remains the gold-standard treatment for FECD.
Inheritance and associations
FECD has been classically described as having an autosomal dominant inheritance. However, the late age of symptom onset and variable penetrance and expressivity of FECD continue to make the determination of the precise mode of inheritance challenging. Indeed, the exact pathogenesis of FECD is still unclear. Still, evidence points to several cellular changes, including alterations in the extracellular matrix, epigenetic modifications, endothelial-mesenchymal transition, cell apoptosis, cellular stress, and the unfolded protein response. The most common genes associated with FECD include SLC4A11, TCF8/ZEB, LOXHD1, AGBL1, TCF4, KANK4, LAMC1 and LINC009970/ATPB1 and specifically for early-onset disease, COL8A2.
There is an association with hypermetropia, with FECD patients typically having shorter axial lengths and shallower anterior chambers than those in control groups. However, the underlying reason for the correlation is not completely understood but could be due to genetic linkage. Notably, the shallow anterior chambers may increase the risk of endothelial decompensation following phacoemulsification of advanced cataract.
Clinical presentation
FECD has a clear bimodal age distribution. The classic form of the disease occurring in middle age has a female predominance with a female-to-male ratio of 3:1. An uncommon form of FECD has onset in the first decade of life and shows a similar progression as the classic phenotype. However, this early-onset FECD affects females and males equally.
Studies suggest that the prevalence of guttae and FECD varies by race and geographic location. A study from Iceland examining a European population >55 years of age found the prevalence of primary cornea guttata to be 11% in females and 7% in males. In contrast, the prevalence is 4% in Japan and the US. However, the incidence of FECD is estimated to be 0.5% in Japan and much higher in the US, where 36% of all corneal transplants performed have a primary indication of FECD. In New Zealand, 18.8% of all transplants are performed for corneal dystrophies. Therefore, it is thought that FECD has a higher prevalence in European populations than in other ethnicities.

Fig 2a. Endothelial specular microscopy showing early FECD with good ECC (>2,000 OU)
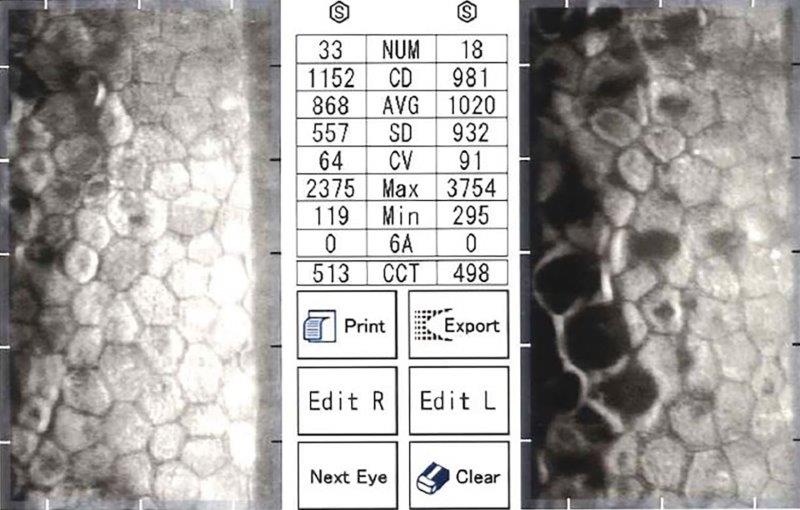
Fig 2b. Endothelial specular microscopy showing moderately advanced, asymmetric
FECD, OS worse than OD with low ECC (OD 1152, OS 981)
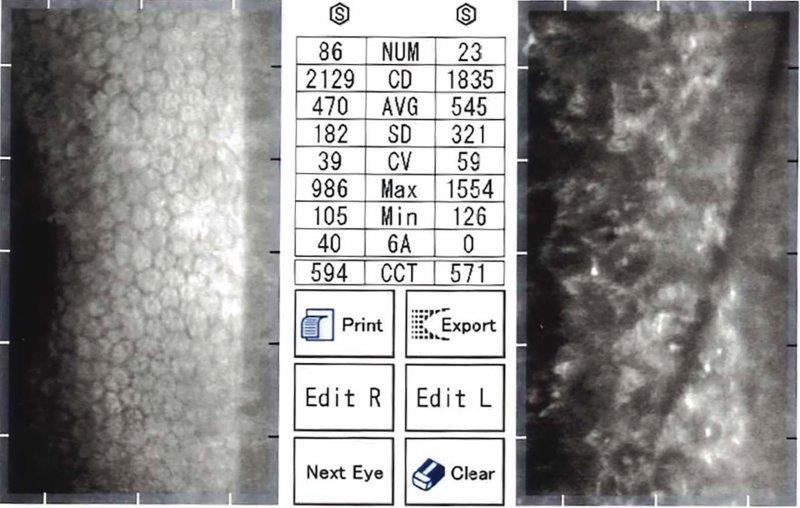
Fig 2c. Endothelial specular microscopy showing extremely advanced FECD, DSAEK OD
(ECC 2129) and untreated OS with almost contiguous guttae
Clinical signs of FECD include corneal guttae, a thickening of Descemet’s membrane, reduction in endothelial cell density with abnormalities of cell shape (pleomorphism) and variations in cell size (polymegathism), as identified on endothelial imaging, such as specular microscopy and confocal microscopy (Fig 2). Late-stage FECD includes corneal oedema and bullae, which can rupture, causing pain, photophobia and reduced vision. Corneal tomography shows an oedematous, thickened cornea (Fig 3).
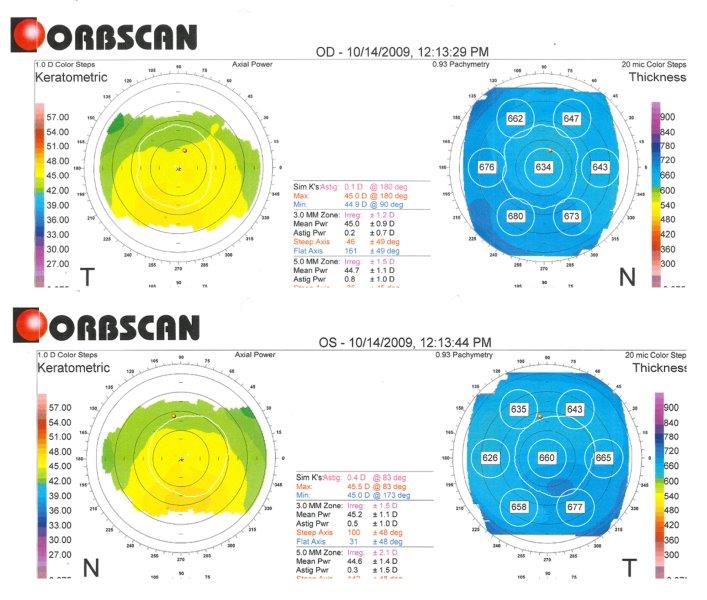
Fig 3. Orbscan computerised corneal tomography of right eye (superior) and left eye (inferior) of study case at presentation (showing keratometric and pachymetry maps), highlighting increased corneal thickness: centrally 634um OD and 660um OS
Management
The current mainstay treatment of visually significant FECD is corneal transplantation. Options include full-thickness penetrating keratoplasty (PKP) in very advanced disease but more commonly Descemet stripping (automated) endothelial keratoplasty (DSAEK or DSEK), or Descemet membrane endothelial keratoplasty (DMEK). Descemet’s membrane and endothelium are removed from the host cornea and are replaced in DS(A)EK with a donor tissue button that consists of Descemet’s membrane, endothelium, plus posterior stroma of variable thickness (~100µm), which compared to DMEK is easier to handle and results in greatly reduced re-bubbling rate. DMEK utilises only Descemet’s membrane and endothelium (~15µm). As a result, the cornea after DS(A)EK is a slightly thicker than a normal cornea (~550µm), which may affect the final outcome but typically only by a line or less of visual acuity (VA).
As most patients develop FECD later in life, they usually have co-existing cataracts, complicating management. Cataract surgery in the setting of FECD can be challenging as intraocular surgery may accelerate endothelial cell loss. Damage is caused in various ways, including mechanical injury, hydrodynamic injury, heat injury, chemical injury, or possibly free-radical formation secondary to high-frequency ultrasound. However, the introduction of viscoelastic material and a protective soft-shell technique has been demonstrated to reduce endothelial damage. A recent study using specular microscopy to image the corneal endothelium in eyes which had previously undergone cataract surgery and intraocular lens implantation demonstrated a significantly lower endothelial cell density (1,825 cells/mm2) relative to eyes without previous cataract surgery (2,400 cells/mm2).
However, it is possible to consider a combined cataract extraction, lens implantation and keratoplasty. Unfortunately, currently there is no consensus in the literature, with combined and staged procedures having similar final VA and endothelial cell count outcomes.
Case example
A 53-year-old European male presented to tertiary care for corneal assessment in 2009 with a five-year history of fluctuating vision, poorer in the morning and improving as the day progressed. His sister had recently been diagnosed with FECD. His VA at referral was OD 6/18-1 with spectacles, improving to 6/9.6 with pinhole, and OS 6/12 with spectacles, not improving with pinhole. He had early cataracts and low hypermetropia (OD +1.50/-0.75x92, OS +1.25/-0.25x98). He exhibited moderate endothelial guttae, left eye worse than right, with pachymetry of 634µm and 660µm, OD and OS, respectively (Fig 3). A diagnosis of FECD was confirmed.
In 2010 he proceeded with time-separated, left phacoemulsification prior to a planned left DSAEK. At one month post-DSAEK review, vision had already improved to 6/7.5 unaided and his intraocular pressure (IOP) was 10mmHg (Fig 4). In 2011, following right phacoemulsification, he also underwent right DSAEK. At his two-month post-DSAEK follow-up, he achieved 6/12 unaided (best spectacle-corrected visual acuity (BSCVA) 6/6).
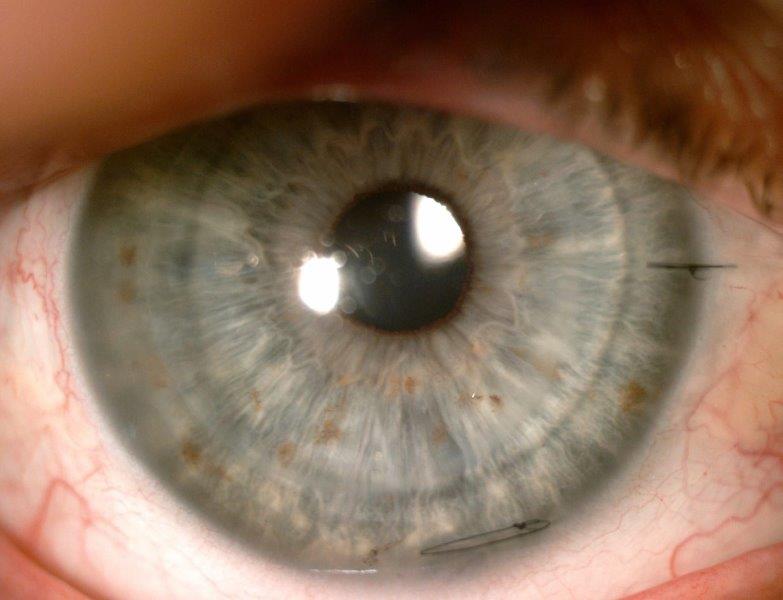
Fig 4. Anterior segment photograph of left DSAEK of study
case at one month follow up, highlighting a clear 8.00mm
transplant with a single scleral and corneal suture
Five years later (2015) he presented with OD 6/7.5 unaided, OS 6/9+2 unaided and it was noticed he had subtle signs of low-grade allograft rejection OD. He was re-commenced on intensive Pred Forte (prednisolone acetate) 1% drops, with the graft rejection quickly resolving to achieve unaided vision of 6/6-1 OD.
In 2022, endothelial specular microscopy, 11 years after right DSAEK and 12 years after left DSAEK, showed very healthy cell counts of 1,314 OD and 1,614 OS with crystal clear corneas and healthy endothelial transplants (Fig 5). For comparison, Price reported median cell loss of 71% and 76% for DSAEK and PKP respectively, relative to donor ECC, at 10 years.
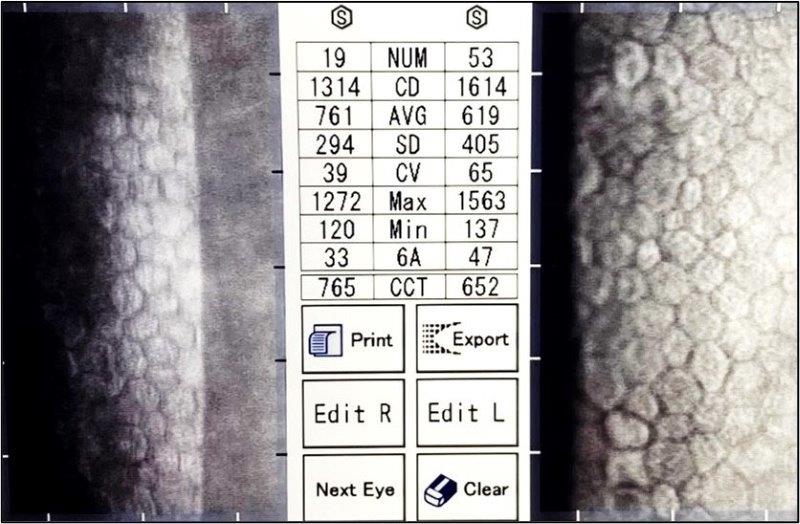
Fig 5. Specular microscopy of clear corneas of study case at more than ten years postsurgery, showing bilateral DSAEK grafted endothelium with healthy, regular, endothelia and ECC density
Discussion
Notably, a study by Chilibeck et al showed corneal dystrophies were the primary indication in 18.8% of all corneal transplants in New Zealand in 2019, of which 92.8% were FECD. Prior to the introduction of endothelial transplants in New Zealand in 2007, PK comprised 91.4% of all transplants, falling to 55% by 2019, whereas endothelial grafts increased from 0% to nearly 40% (29.5% DSAEK and 9.1% DMEK) in the same period.
The case example conforms to the classic presentation of FECD, with first symptoms starting in his 40s, positive family history and mild hypermetropia. On examination, he had corneal endothelial guttata and mild cataracts. Therefore, he underwent phacoemulsification with intraocular lenses a few weeks prior to DSAEK to minimise endothelial damage.
Unfortunately, corneal transplantation carries a risk of post-operative rejection (as in this case) and failure is limited by donor tissue availability – a major issue in Aotearoa over the last two years. Fortunately, the allograft rejection rate is lower in endothelial transplants than penetrating keratoplasty.
Recently, other novel treatment modalities have been studied, such as Rho-associated protein kinase (ROCK) inhibitors and self-healing. Ripasudil hydrochloride hydrate (Glanatec ophthalmic solution 0.4%, Kowa Co Ltd), a ROCK inhibitor used in the treatment of glaucoma, has been shown to reactivate cell proliferation and migration as well as restoring endothelial pump and barrier function without inducing adverse phenotypic changes in FECD corneas.
Some studies have investigated the possibility of ‘self-healing’ corneas, whereby diseased Descemet’s membrane and endothelium are removed, and peripheral stem-like endothelial cells migrate to regenerate the endothelium (with or without ROCK inhibitors). This procedure, Descemet’s stripping only/Descemetorhexis without endothelial keratoplasty (DSO/DWEK), in limited studies, has shown to have a complete but slow resolution of corneal oedema in 63-100% of cases. Initial studies at Eye Institute, Auckland, demonstrated the transplant-free DSO/DWEK procedure with ROCK inhibitors is associated with intense oedema in the central 4mm of the cornea for a number of weeks but VA of 6/9 can be regained within three months.
As always, patients need to be evaluated individually and the treatment tailored to their disease. However, it is apparent that endothelial transplant is now far more popular among surgeons than full-thickness transplantation for FECD, due to more stable refractive outcomes and more rapid visual recovery. DSO/DWEK may also have a future role in the subgroup where FECD is primarily central with relatively normal peripheral endothelium. All eyecare practitioners await the further development of novel techniques in the next 5-10 years to obviate transplantation and avoid associated rejection risks.
Bibliography
1. Sarnicola C, Farooq A, Colby K. Fuchs’ endothelial corneal dystrophy: update on pathogenesis and future directions. Eye & Contact Lens. 2019 Jan 1;45(1):1-0.
2. Pitts, J, Jay, J. The association of Fuchs’ corneal endothelial dystrophy with axial hypermetropia, shallow anterior chamber, and angle closure glaucoma. British Journal of Ophthalmology. 1990 74; 601–604.
3. Chilibeck C, Brookes N, Gokul A, Kim B, Twohill H, Moffatt S, Pendergrast D, McGhee C. Changing trends in corneal transplantation in Aotearoa/New Zealand, 1991 to 2020: effects of population growth, cataract surgery, endothelial keratoplasty, and corneal cross-linking for keratoconus. Cornea. 2022 Jun 2;41(6):680-7.
4. Ahmed K, McLaren J, Baratz K, Maguire L, Kittleson K, Patel S. Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. American Journal of Ophthalmology. 2010 Oct 1;150(4):490-7.
5. Moshirfar M, Huynh R, Ellis J. Cataract surgery and intraocular lens placement in patients with Fuchs corneal dystrophy: a review of the current literature. Current Opinion in Ophthalmology. 2022 Jan 1;33(1):21-7.
6. Schlötzer-Schrehardt U, Zenkel M, Strunz M, Gießl A, Schondorf H, Da Silva H, Schmidt G, Greiner M, Okumura N, Koizumi N, Kinoshita S. Potential functional restoration of corneal endothelial cells in Fuchs endothelial corneal dystrophy by ROCK inhibitor (Ripasudil). American Journal of Ophthalmology. 2021 Apr 1;224:185-99.
7. McGhee C, Zhang J. Considering the evidence base in corneal transplantation: A penetrating, lamellar or cellular future? Clin Exp Ophthalmol. 2022 May;50(4):371-373.
8. Jeang L, Margo C, Espana E. Diseases of the corneal endothelium. Experimental Eye Research. 2021 Apr 1;205:108495.
For more, see https://www.nzoptics.co.nz/articles/archive/long-term-trends-in-keratoplasty-in-aotearoa and www.nzoptics.co.nz/articles/archive/corneal-donation-and-corneal-transplantation-in-aotearoa

Dr Lize Angelo is an HRC clinical research fellow in the UoA Ophthalmology Department, headed by Professor Charles McGhee. She’s pursuing a PhD in diagnosing and treating keratoconus, including aspects of equity of access to services in Aotearoa.

Dr Akilesh Gokul, a senior lecturer in the UoA Ophthalmology Department, is an optometrist and clinician-scientist specialising in contact lenses at Mortimer Hirst Optometrists. He also works in Te Whatu Ora Auckland’s crosslinking service. His clinical and research interests include keratoconus, contact lenses, crosslinking and inequity in healthcare.