Managing glaucoma – a journey of faith and patience
Glaucoma is a chronic progressive disease and the lack of clear targeted guidelines to match every patient’s need makes its management challenging. The unpredictability of response to various treatment modalities makes things trickier still. Rather than being the end of the treatment spectrum, surgery creates a different pathway towards the goal. Secondary glaucoma adds the challenge of not only controlling the intraocular pressure (IOP) but also taking care of the root cause, which may be inevitable. This case of recurrent conjunctival erosion following a glaucoma implant represents my journey with my patient, creating a bond of faith.
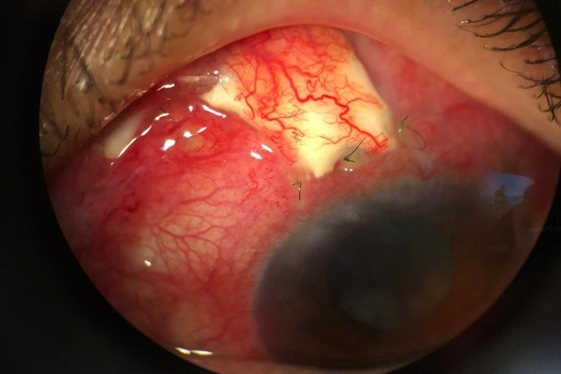
A wāhine Māori presented to the acutes clinic with type-1 uncontrolled diabetes and a painful, red left eye. Clinical examination revealed a steamy cornea with 360° neovascularisation of the iris and angle and IOP of 62mmHg. Visual acuity was 6/12 in the right eye and perception of light in the affected left eye. She had bilateral cataracts and fundus examination showed bilateral proliferative diabetic retinopathy (although the view was hazy in the left eye due to the steamy cornea). Treatment was intravenous acetazolamide, which instantaneously reduced IOP to 48mmHg. Left eye intravitreal Avastin (bevacizumab) was administered with paracentesis and IOP-lowering drops. A review after three days showed a hyphaema and an IOP of 42mmHg on maximal medication, including oral acetazolamide. Fundus examination showed advanced optic disc damage with a disc damage likelihood scale (DDLS) 8 in the left eye and moderate disc damage, DDLS 6, in the right eye. She underwent bilateral pan-retinal photocoagulation for proliferative diabetic retinopathy.
Followed-up a week later, although the hyphaema had resolved with a regression of the neovascularisation in the left eye, her IOP was 42mmHg on maximal medication. She underwent left Ahmed glaucoma valve implant with an Amniotek-G membrane, which was uneventful. Postoperative day one revealed a beaming and happy patient with vision of 6/12 and an IOP of 8mmHg in the left eye. She continued on topical steroids and cycloplegics and a follow-up visit was planned in two weeks.
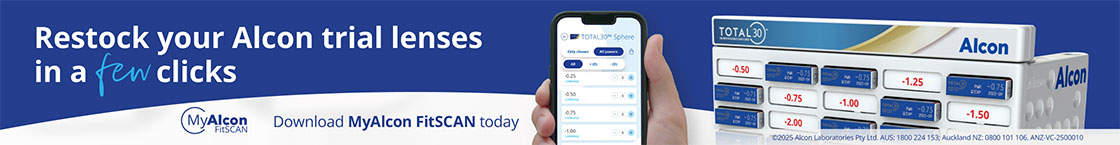
However, she returned after 10 days with a history of gritty sensation in the left eye and admitted to having rubbed it vigorously. Although the vision and IOP was maintained in the left eye, there was a retraction of the conjunctiva at the limbus, with broken sutures exposing the tube of the glaucoma implant. She was taken back to theatre where a very friable conjunctiva was sutured back. She continued with the same drops and at four-week follow-up presented with conjunctival erosion and an exposed tube of the glaucoma implant (Fig1). She maintained vision of 6/12 and IOP of 8mmHg in the left eye.
Fig 1. Conjunctival erosion with exposure of the tube of the Ahmed glaucoma valve implant
We had a discussion regarding the risk of endophthalmitis and she underwent a scleral patch graft with conjunctival autograft the same day. She did well for two months, with good vision and IOP, but again presented with conjunctival tube erosion. The overlying sclera and conjunctiva had eroded but the plate was well encapsulated and the IOP was maintained at 8mmHg without drops. Following discussion with my colleagues in Auckland, she was scheduled for replacement of the Ahmed glaucoma implant with a Baerveldt tube and Supramid ripcord suture. Postoperatively, she was unhappy as her vision had dropped to perception of light due to a half-chamber hyphaema and her IOP was at 44mmHg on four drops per day.
Déjà vu
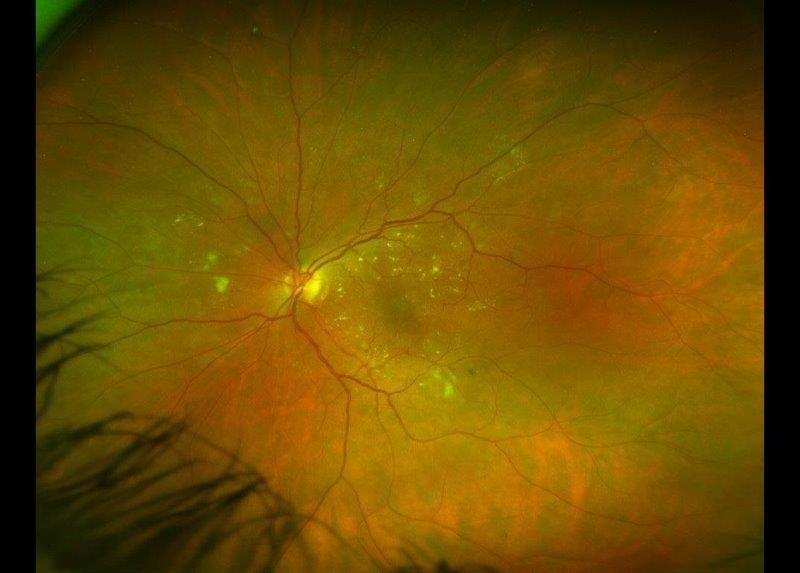
A week later, we breathed a sigh of relief to see the hyphaema had resolved and the IOP had dropped to 36mmHg… but we were totally unaware of what was coming! She walked back into the clinic with a red sore eye three days later and this time the conjunctiva overlying the plate of the Baerveldt implant had eroded, exposing the implant plate (Fig 2). The implant had to be removed urgently and we were able to offer nothing further at that stage. Hence, the fibrosed conjunctiva was sutured back in the best possible way to prevent any aqueous leak.
Although her IOPs were being monitored, her frustration was understandably severe and I was unable to offer anything other than reassurance. After discussion with many colleagues regarding micropulse transscleral photocoagulation to reduce her IOPs, the prevailing opinion was to leave her alone as the left eye was too compromised. Her GP was also involved and an improving glycaemic level was the only light at the end of the tunnel.
A month later, she was still unhappy as her vision in the left eye was perception of light and her IOP was 42mmHg on four daily drops with a closed angle. The cataract had progressed with advanced disc damage. After an open discussion, left-eye cataract surgery with goniosynechiolysis was performed under guarded visual prognosis. The lady who came in the next day was a totally different person. Confident and radiant she walked up to the nurses’ station, where they recorded unaided vision of 6/12 and IOP of 18mmHg. The anterior chamber was deep, with a 0.5mm hyphaema, so she was continued on topical steroids and cycloplegics. Four weeks later, the vision in the left eye had improved to 6/9, pinholing to 6/6, while her IOP was 8mmHg without drops. Vision was still 6/12 in the right eye, which she now felt she was not seeing well from. She had a grade-2 nucleosclerotic cataract in this eye and was advised to schedule cataract surgery and visit the dispensing optician for glasses.
A six-monthly review was planned but she returned four months later with a painful red right eye. Vision had dropped to perception of light in the right eye and IOP was 58mmHg with neovascularisation of the iris, despite extensive pan-retinal laser photocoagulation (PRP). She received intravitreal Avastin, an interim PRP and she was started on all pressure reducing drops. The left eye maintained a vision of 6/9 and IOP of 10mmHg. Her right eye received the Ahmed glaucoma valve implant and, pleasingly, her IOP dropped to 8mmHg day one postoperatively. Two months later, my journey with this patient continues. She maintains vision of 6/6 in both eyes with glasses. Her IOP is in the low teens bilaterally and the diabetic retinopathy remains stable. We keep our fingers crossed as we wait to see what the future holds.
Discussion
Glaucoma drainage devices (GDDs) are associated with various complications, such as tube migration, tube or plate exposure or extrusion, ocular motility disturbance and infection¹. Tube exposure through eroded conjunctiva is one of the more severe complications of GDD surgery². The rate of conjunctival tube erosion (CTE) after GDD surgery ranges from 0.8-6.3% and is a risk factor for endophthalmitis³. Determinants for CTE include age, female gender, ethnicity, orbital dimension, presence of health comorbidities, diagnosis, chronic use of topical medications, number of previous and concomitant ocular surgeries and type of patch graft used⁴-⁷.
Muir et al showed an increased chance of developing CTE among females and postulated that postmenopausal hormonal imbalance and a disparity in orbital dimensions between males and females are the primary reasons for females’ increased risk⁸. The smaller orbital dimensions in females is considered a cause for additional microtrauma and friction between the eyelid and ocular tissues overlying the tube.
Several theories attempt to account for CTE development. These include chemical, anatomical, immunologic or mechanical factors, such as micromovements of the tube during eye movements and blinking, and degenerative changes in the conjunctival tissue and Tenon’s capsule⁹-¹³. We attributed our patient’s recurrent tube erosion to female gender and uncontrolled diabetes. Her use of an increased number of drops and smaller orbital dimensions could also have contributed to micromovements of the tube during blinking, possibly resulting in recurrent erosion of the tube. Similar to Smith, we found no statistically significant difference when comparing CTE incidence among our patients where an autologous patch graft, donor sclera or processed pericardium was used to cover the tube, since the tube eroded irrespective of the Amniotek-G or the scleral patch graft.
To conclude, females and diabetics are at risk of developing CTE. An increased number of postoperative IOP-lowering eye drops and increased number of major intraocular surgeries also contribute to CTE. These risk factors should be taken into consideration when performing glaucoma valve implantation.
References
- Shrivastava & Jeffery (2011). Recurrent Conjunctival Erosion After Glaucoma Tube Implantation. Glaucoma today: challenging cases
- Minkler (2008). Aqueous shunts in glaucoma AAO OTA. Ophthalmol. 115:1089-1098.
- Dubey, Prasanth & Acharya (2013).Conjunctival erosion after glaucoma drainage device surgery: A feasible option. Indian Ophthalmol. Jul; 61(7): 355–357.
- Muir et al (2014). Risk factors for exposure of glaucoma drainage devices: a retrospective observational study. BMJ Open. 4:e004560
- Koval et al (2013). Risk factors for tube shunt exposure: a matched case-control study. Ophthalmol. 196215
- Byun, Lee & Park (2009). Risk Factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantation. Japan Ophthalmol. 53:114-19
- Trubnik et al (2013). Evaluation of the risk factors for glaucoma drainage device-related erosion: A retrospective case control study. Glaucoma. 00:1-5. 17
- Ferrario et al (2001). Morphometry of the orbital region: a soft tissue study from adolescence to mid-adulthood. Plast Reconstr Surg. 108:285-93.
- Oana (2012). Tube exposure repair. Current Glau Prac. 6:139-142.
- Lankaranian (2008). Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery. Glaucoma. 17:48-51.
- Smith (2002) A comparison of glaucoma drainage implant tube coverage. Glaucoma. 11:143-147
- Pakravan et al (2001) Superior versus inferior Ahmed glaucoma valve implantation. Ophthalmol. 108:1323-1327
- Heuer, Budenz & Coleman (2001). Aqueous Shunt tube erosion. Glaucoma. 10:493-496.

Dr Baswati Sahoo, a retired airforce officer, is a cataract and glaucoma surgeon with 14 years’ experience in India and Abu Dhabi. She practices at Eye Institute Hawke’s Bay.