NZ team’s keratoconus drops set to replace surgery
The team behind the University of Auckland (UoA) spin-out TheiaNova claim they are close to treating keratoconus and possibly myopia with Pachymatrix, a new cornea-strengthening eye drop.
PhD student Carol Greene, working with faculty members Professor Emeritus Colin Green, Professor Trevor Sherwin and Associate Professor Ilva Rupenthal, discovered corneal keratocytes could be reprogrammed to produce a type of collagen normally only expressed in the embryonic stage of development. This collagen can restore the rigidity lost in keratoconic corneas, the team said.
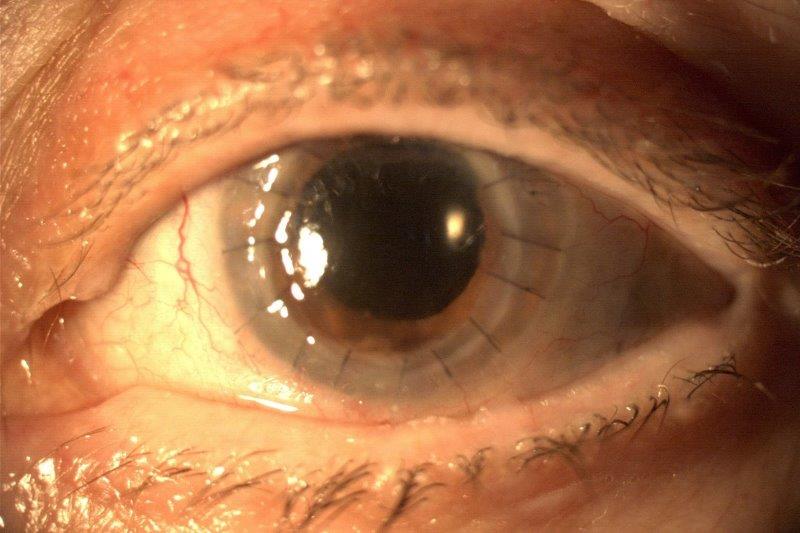
Until the advent of corneal crosslinking (CXL), keratoconus accounted for more than 40% of New Zealand’s corneal transplant cases, the highest reported proportion of transplantation surgery for keratoconus worldwide. “CXL is the gold standard and will remain so for some time yet,” said Prof Green. “However, it is a surgical procedure and for younger patients, and the evidence is that it will require more than one treatment over time. Pachymatrix is an eye drop twice a day for three to six weeks.”
The treatment would be effective for patients using an orthokeratology (ortho-k) lens, he said. “By placing an eye drop into the eye and wearing the lens overnight and treating again in the morning, we expect to hold the cornea back while it stiffens. That will not only prevent disease progression, but significantly improve vision.” Although keratoconus has been the team’s focus, Prof Green said Pachymatrix’s mechanism may also be useful to tackle myopia. “Many myopia patients already wear ortho-k lenses. The cornea gradually reverts to its natural shape, but if we can treat with Pachymatrix to hold the flattened position, the patient may be able to forego the lenses.”
UoA’s commercialisation company UniServices supported the team with their initial due diligence and helped secure the intellectual property (IP) rights. Bridgewest Ventures NZ then stepped up to provide backing for the first human study. TheiaNova CEO Carissa Fonseca said if the study is successful, Pachymatrix will start the five-to-ten-year pathway for regulatory approval. “Later phases of clinical research require recruitment of large numbers of patients, which can take some time for a hard-to-diagnose disease like keratoconus. However, we’re working to a very ambitious timeline. We want to do this as quickly as we can because this could be a game changer for patients,” she said.