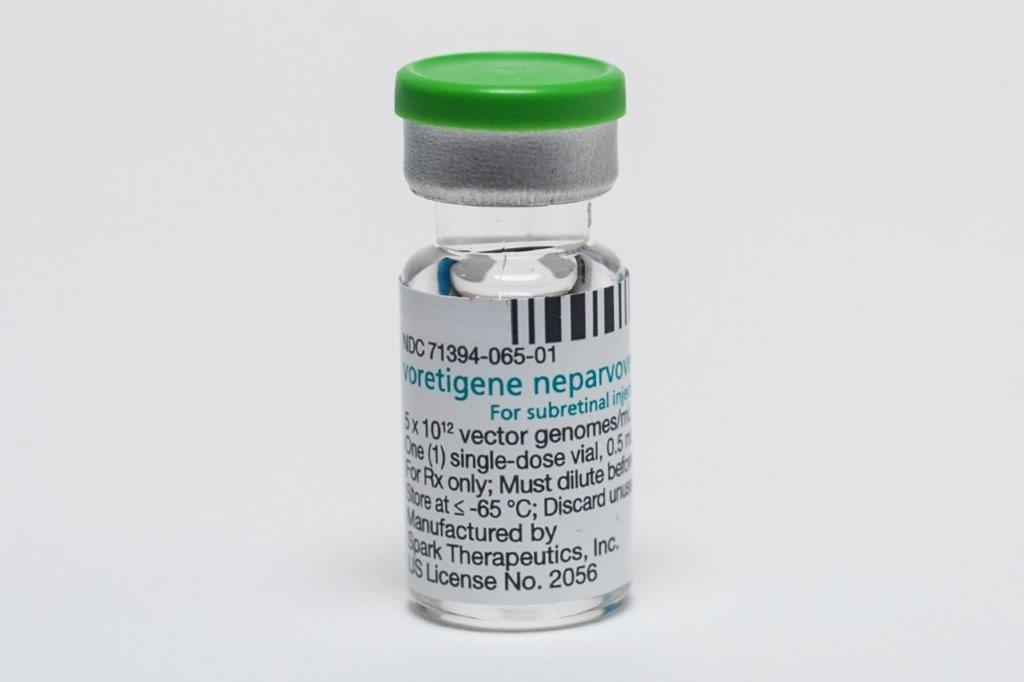
Medsafe approves Luxturna
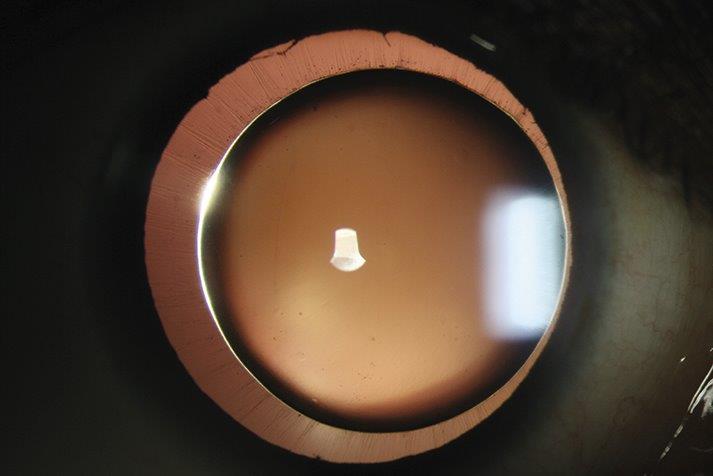
Years of campaigning, led by consultant ophthalmologist Associate Professor Andrea Vincent from the University of Auckland, have paid off with the Medicines and Medical Devices Safety Authority’s (Medsafe’s) approval of Luxturna (voretigene neparvovec 0.05mg/mL) to treat patients with RPE65-related Leber congenital amaurosis (LCA) in New Zealand.
Since Medsafe approved the breakthrough gene therapy late last year, A/Prof Vincent said she has met with Sean Evans, NZ medical head of Novartis (which has the development and commercialisation rights to Luxturna outside the US) to help drive the application to secure Pharmac funding for the one-time treatment. A/Prof Vincent said there are three patients currently eligible for Luxturna treatment in New Zealand and though it’s incredibly expensive – costing about $675,000 per eye, excluding surgical expenses – multiple analyses show the cost benefits and improvement in quality-adjusted life-years for those treated.

Consultant ophthalmologist and retina specialist A/Prof Andrea Vincent
Pharmac said Luxturna’s application status is currently ‘seeking clinical advice’ but this will be reviewed at the next Pharmacology and Therapeutics Advisory Committee meeting on 17 February 2023. A/Prof Vincent said she is hopeful the committee will recognise the impact of funding Luxturna, noting that Pharmac has recently shown a commitment to funding medications for rare diseases, such as cystic fibrosis and spinal muscular atrophy. Luxturna’s approval could also bring broader benefits to New Zealanders, she said. “Although this is a rare disease, the process of establishing the requirements for delivery in New Zealand mean that many other patients will potentially benefit from the other gene treatments in advanced clinical trials, and we may also be able to now participate in phase 3 clinical trials.”
Luxturna was approved for use in the US in 2017, the EU in 2018 and Australia in August 2020, with the first vision-improving operations carried out in late 2020 and early 2021 in Sydney.