IRD gene therapy advances
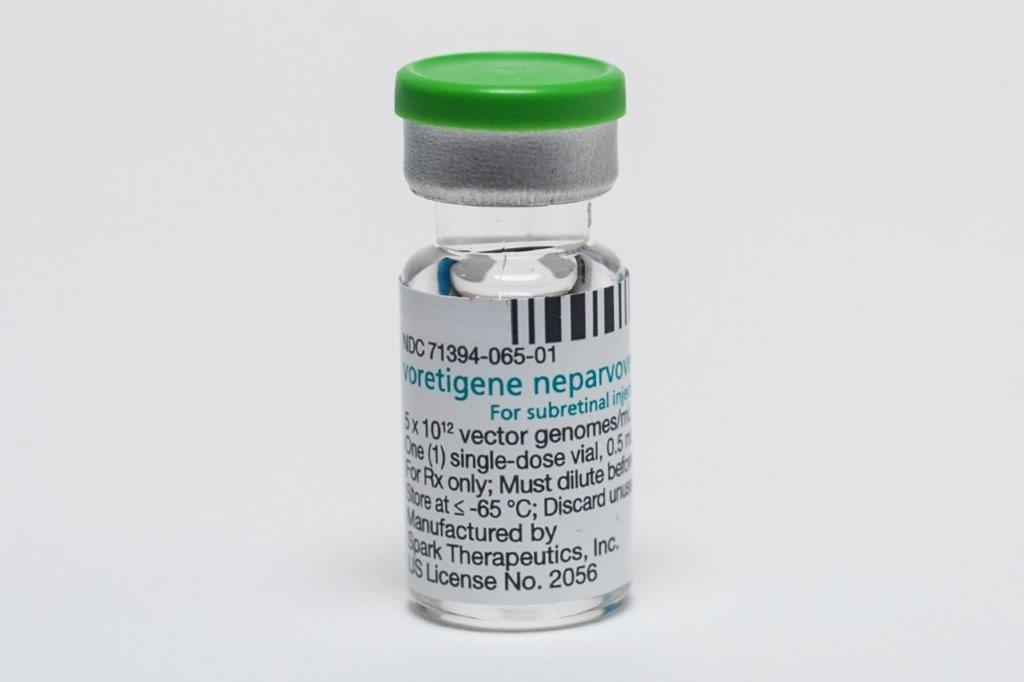
On the heels of Luxturna’s (voretigene neparvovec-rzyl) Medsafe approval in New Zealand to treat RPE65-mediated Leber congenital amaurosis (LCA), several other gene therapies for inherited retinal diseases (IRDs) are on the horizon.
GenSight: GS030-MD for RP
France’s GenSight Biologics announced two of nine late-stage retinitis pigmentosa (RP) patients achieved a significant improvement in vision a year after treatment in its phase 1/2 Pioneer clinical trial of GS030-MD. The two patients were barely able to perceive light before treatment but at one year were able to locate and count objects, reported Professor Jose-Alain Sahel from Sorbonne Université.
GS030-MD delivers a light-sensitive protein, chrimsonR, to retinal ganglion cells. Treatment includes goggles which project light pulses onto the retina to activate the cells.
SparingVision: SPVN06 for RP
Also based in France, SparingVision announced US Food & Drug Administration (FDA) clearance for its new drug application for SPVN06, another gene therapy for RP. France’s National Agency for the Safety of Medicines and Health Products is also reviewing the company’s application for clinical trials of SPVN06, which is aimed at stopping or slowing disease progression in patients affected by IRDs and dry age-related macular degeneration (AMD), regardless of their genetic background.
“With over 80 genes involved in RP, each with numerous causative mutations, we need to go beyond the gene-by-gene treatment approach. SPVN06 has the potential to become the universal therapeutic solution that patients need, and we are excited for the next phase of development,” said Stéphane Boissel, SparingVision president and CEO. The company raised €75m ($123.9m) in series B financing for genomic medicines in 2022.
Opus Genetics: OPGx-001 for LCA
In the US, biotech firm Opus Genetics announced the FDA cleared in-human trials of OPGx-001, a viral-vector which delivers functional LCA5 genes to photoreceptors in patients with LCA5-mediated LCA. Nine adults will participate in the dose-escalation trial, with a paediatric cohort being added if it is shown to be safe. If successful, OPGx-001 will be the first treatment for this LCA variant.