The damage that drops can do!
Ocular surface disease (OSD) is a multifactorial disorder of the conjunctival epithelium, cornea, lacrimal and meibomian glands resulting in either deficient or inappropriate tear production. This can decrease visual acuity (VA) and result in significant ocular discomfort1. OSD can occur in conjunction with many other ocular conditions and often co-exists with glaucoma.
At present, 11% of the five million Americans over 50 who have dry eye disease (DED) also have glaucoma2. Topical medical therapy is the most common initial treatment for glaucoma and 49-59% of glaucoma patients on such medications have some form of OSD3. OSD in these patients can be a pre-existing condition that is exacerbated by topical therapy or a novel disease that manifests after initiation of topical glaucoma therapy. Topical glaucoma medications can cause significant morbidity, with patients complaining of a burning sensation, irritation, itching, tearing, skin pigmentation and decreases in VA, often within three months of medication initiation4.
Furthermore, untreated primary open angle glaucoma (POAG) patients have a higher risk of OSD, in part due to a 22% lower basal tear turnover rate in comparison to patients without glaucoma5. The resulting OSD symptoms in patients with glaucoma can lead to poor medication compliance. Cessation of therapy by the patient without informing the physician can result in elevated IOP and disease progression.
OSD is also linked to a higher rate of failure in filtration glaucoma surgery. Thus, management of OSD in glaucomatous patients is important when trying to reduce further ocular morbidity and to improve the success of glaucoma therapy.
Examining cases
A 69-year-old female patient with unilateral POAG presented complaining of chronic conjunctival injection and irritation. Her condition was stable, based on serial visual field testing and OCT analysis of the optic nerves. Her IOP was 18mmHg with Goldmann applanation tonometry (GAT). Her glaucoma was being treated with latanoprost nocte in the left eye. On this last review she was found to have developed a cataract and was keen to explore her options for reducing or eliminating the drop burden on her ocular surface. Clinical examination revealed significant skin pigmentation and conjunctival injection of the left eye (Figs 1 and 2).
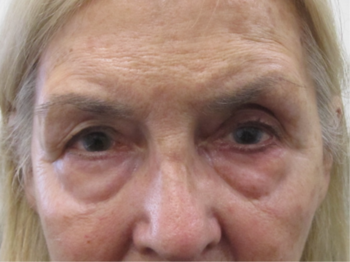
Fig 1. Patient showing significant skin pigmentation due to chronic prostaglandin analogue use in the left eye. There is mild enophthalmos with deepening of the sulcus superiorly
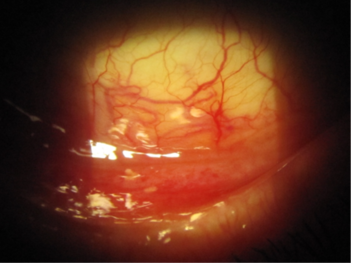
Fig 2. Significant conjunctival injection due to chronic prostaglandin use in the inferior fornix of the left eye
Cataract surgery was performed in combination with implantation of the iStent inject system in the trabecular meshwork. Her postoperative IOP was 14mmHg and the latanoprost drops were ceased in the left eye. Within a month there was a marked improvement in the appearance of her conjunctiva with resolution of the symptoms of irritation. Her forniceal conjunctiva ceased to be injected (Fig 3).
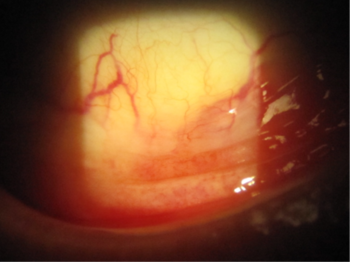
Fig 3. Image of the left conjunctival fornix indicating marked improvement in the appearance of the conjunctiva with resolution of the erythema
The chronic use of IOP-lowering medications can have other unintended consequences on ocular surface management. The use of prostaglandin analogues has also been associated with local tissue atrophy in the orbit (a type of orbitopathy) reported to occur with several prostaglandin analogues6. This can result in deepening of the orbital sulcus due to the loss of adipose and connective tissue in that area (Fig 4 and 5).
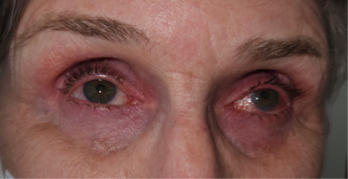
Fig 4. Prostaglandin-related orbitopathy. Note the sunken appearance of both eyes with deepening of the superior sulcus. This patient was receiving bimatoprost for glaucoma prior to iStent surgery
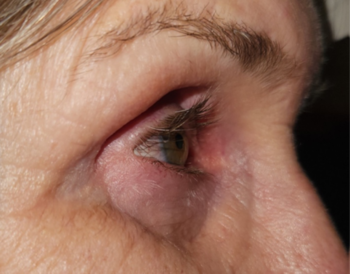
Fig 5. Lateral view of the same patient indicating the resultant tissue atrophy from chronic PGA use. In some cases the degree of enophthalmos can impair Goldmann applanation tonometry as the eyes sink into the orbit
Preservative-induced inflammation
Corneal toxicity secondary to chronic medication use is another area where interventions, such as the iStent inject system, can make a significant difference in eliminating the gritty and stinging sensation associated with drop instillation. The main culprit in these cases is the preservative benzalkonium chloride (BAK).
BAK has been shown to strongly induce the expression of inflammatory mediators in the lens epithelial cells, compared with latanoprost or timolol. The Blue Mountains Eye Study and Ocular Hypertension Treatment Study both suggested higher rates of cataract formation in those receiving anti-glaucoma therapy. Miyake conducted studies that suggested use of BAK-preserved drops prior to cataract surgery increased the risk of cystoid macular oedema⁷. Chronic BAK exposure has been associated with significant ocular surface toxicity, often manifesting as a diffuse punctate keratopathy (Fig 6). With BAK eliminated from the ocular surface following minimally invasive glaucoma surgery (MIGS), significant improvement of the overall corneal health can be seen, with resolution of the signs (Fig 7).
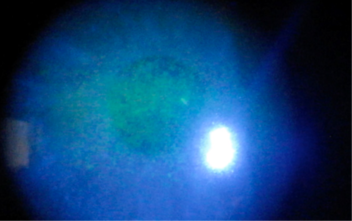
Fig 6. Toxic epitheliopathy in a patient prior to iStent surgery. The patient was on a preserved prostaglandin analogue. Fine punctate erosions of the cornea seen centrally

Fig 7. Two months post-surgery there is a marked improvement in the appearance of the corneal epithelium as exposure to BAK has ceased
In conclusion, the use of microtrabecular shunts, such as the iStent inject system, has been revolutionary in the management of mild to moderate stable glaucoma. The chronic use of drops has a detrimental effect on the ocular surface of these patients, resulting in secondary morbidity which can be significant but can also result in poor adherence to management protocols. It is therefore advantageous to offer this cohort of patients access to MIGS options to reduce or eliminate the drop burden and achieve better outcomes and disease management.
Disclosure: This article was independently written by Dr Alex Ioannidis, who was sponsored by Glaukos
References
- Ramli N, Supramaniam G, Samsudin A, Juana A, Zahari M, Choo M. Ocular surface disease in glaucoma: Effect of polypharmacy and preservatives. Optom Vis Sci. 2015; 92(9):e222–6.
- Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90(3):262–267.
- Leung E, Medeiros F, Weinreb R. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008; 17(5):350–355.
- Rossi G, Scudeller L, Rolle T, Pasinetti G, Bianchi P. From benzalkonium chloride-preserved latanoprost to polyquad-preserved travoprost: A 6-month study on ocular surface safety and tolerability. Expert Opin Drug Saf. 2015; 14(5):619–623.
- Kuppens E, van Best J, Sterk C, de Keizer R. Decreased basal tear turnover in patients with untreated primary open-angle glaucoma. Am J Ophthalmol. 1995; 120(1):41–46.
- Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22:626–631
- Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002 Aug;47 Suppl 1:S203-18.

Dr Alex Ioannidis is a cataract and anterior segment surgeon consulting at South Australia’s Vision Eye Institute. He has been performing combined cataract surgery with the iStent since 2014 and supports the training of surgeons to use the implant.